🕒 4 min
Bubbles are generally fun to make and play with, but did you know you could witness some interesting physics by heating one up? You can watch mini cyclones form and dissolve. Try simulating a hurricane on a bubble in your kitchen!
What you will need
- 150 mL bubble mixture
- 200 mL container (or bigger)
- 25 mL detergent
- 125 mL water
- electric stove
- metal cup
- straw
- flashlight
If you don’t have pre-made bubble mixture, you can make your own using the steps below. If you do, feel free to skip ahead.

1. Add detergent to your container. Use as much as you think you’ll need. Here we used about 25 mL, which is enough to still have leftover mixture after a series of experiments.

2. Slowly add in the water. Be careful not to pour it in too fast because you want to avoid foam or bubbles at this stage. The total amount of water should be five times greater than the amount of detergent. For us, that meant 125 mL of water.

3. Gently stir until combined. Use a chopstick or the handle of a spoon.
If you want to use the mixture later, you should let it sit overnight. Otherwise, it’s perfectly fine to use right away.
Now that you have everything you need, here’s the fun part:

1. Place a metal cup or container upside down on the stove.

2. Pour some bubble mixture on top.

3. Use a straw to blow a bubble. Then turn on the heat and watch what happens.
The results will be the most visible if you dim the lights in your kitchen and shine a white light on the bubble directly. If you still can’t tell what’s going on, try projecting the image of the bubble onto a wall with a flashlight.
Be careful not to burn yourself or accidentally inhale the bubble mixture!
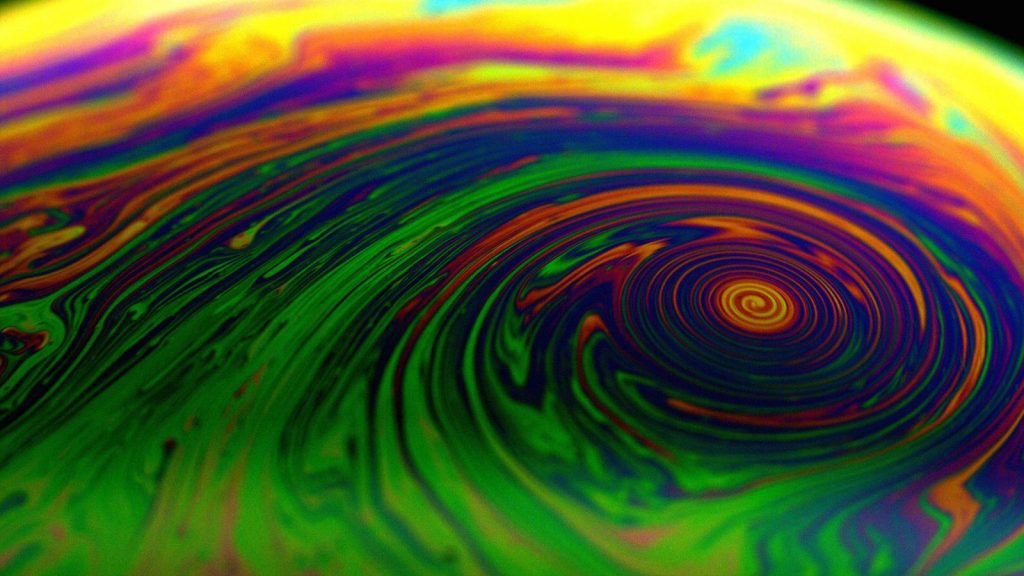
If you watch the bubble as it’s heating up, you’ll see its typical colorful pattern contort into interesting-looking vortices that might remind you of a hurricane! This is difficult to capture without a professional camera setup, but here’s what you might see if you try it yourself:

If you try the projection method, you will mostly see currents inside the bubble film as the heat rises through it.
The warmer liquid from the bottom of the half bubble rises and slowly cools, while liquid from the cooler part of the bubble moves downwards and heats up. This is a great example of convection – these are the currents you’ll see if you project the bubble onto a wall. Bubbles form because detergent molecules are amphiphilic, meaning they have one end that attracts water, while the other repels it. When you introduce a bit of air into the bubble mixture, a thin film forms, with water trapped between two layers of detergent. When the mixture moves within the bubble, its molecules interact and drag on each other. When you heat a half bubble from below, these interactions cause convective currents inside it to swirl when they reach the top, producing a cyclone-like vortex.
You might think that heating the bubble might make it pop. However, if you don’t do it too fast or agitate the bubble in some other way, that may not be the case. Bubbles pop even if you don’t touch them because of gravity and evaporation. Gravity gradually pulls the liquid down and thins out the top of the bubble until it can no longer hold itself together. Additionally, water slowly evaporates until the same thing happens. When you’re heating the bubble, water evaporates faster, but convection is lessening the effect of gravity. Combined, these changes mostly cancel out.
This effect isn’t just pretty to look at – it has also found its uses in atmospheric physics. The behavior of these swirls and currents doesn’t just look like a cyclone, it directly mimics one. In a paper you can read here, scientists compared vortices on bubbles to cyclones in Earth’s atmosphere and found remarkable similarities between them. It turns out that studying soap bubbles can give us useful insight into how real tropical storms evolve, specifically how to tell when they might die out.
You can also watch a video on their research here.
Have you tried making your own soap bubble cyclones? Did you like this experiment? Would you like to see similar ones in the future? Let us know in the comments!
Try seeing if a different bubble mixture might affect the appearance or longevity of the vortices! Try adding 10 mL of glycerin (or, alternatively, corn syrup) to the bubble mixture above. What if you use bubbles of different sizes or multiple bubbles? How would faster or slower heating affect the result? Try it and tell us what you find out!